
科研绘图sci画图作图学术杂志封面设计toc示意图文章配图医学动画
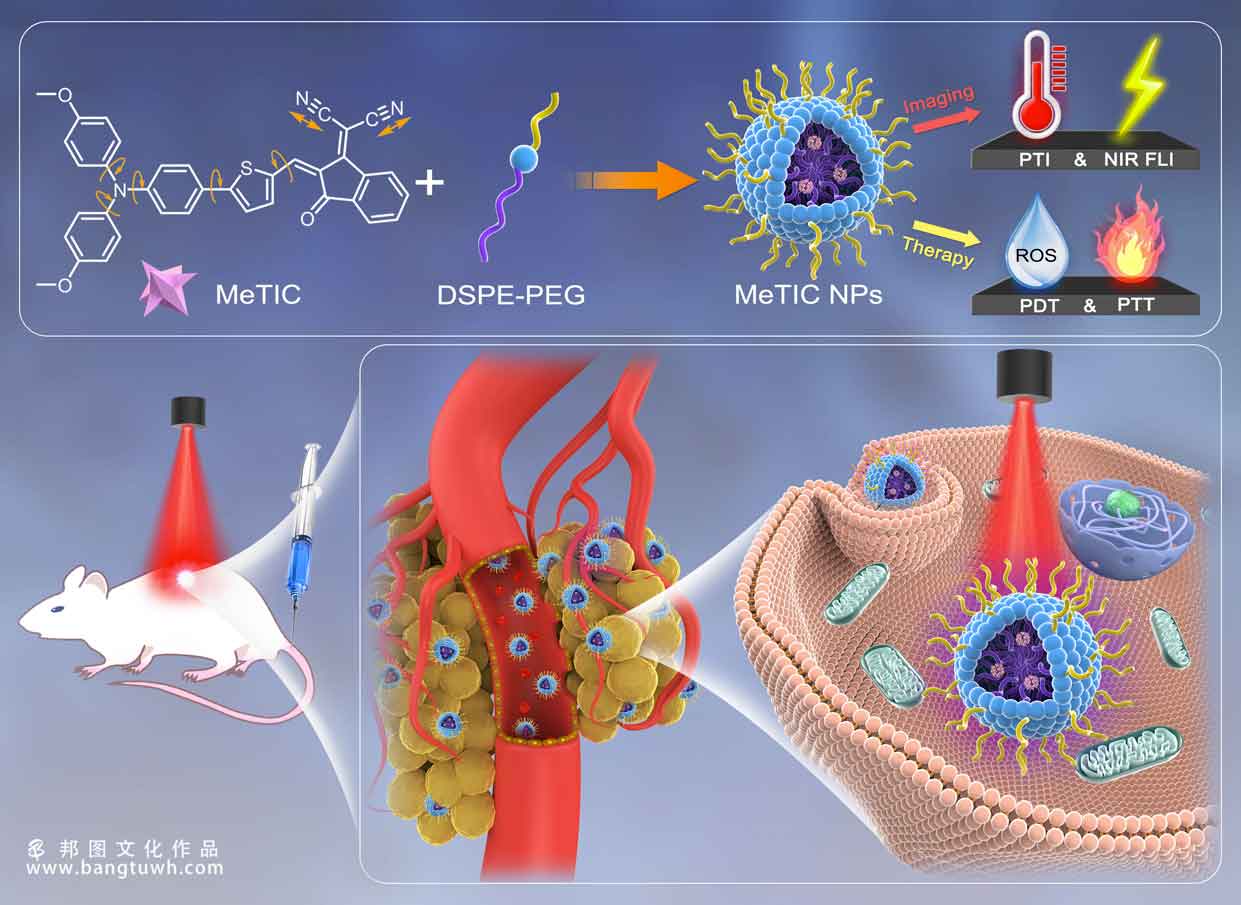




precise molecular engineering is the most fundamental and even a great challenging task for the development of small organic fluorophores used as phototheranostic agents in multimodal imaging-guided synergistic therapy. to the best of our knowledge, there have been no previous reports regarding the fine fabrication of molecular structure from a proof-of-concept study, providing a single molecule with all phototheranostic modalities. herein, an electron donating–accepting (d–a) system is constructed by using triphenylamine derivatives as donors and diverse electron-deficient partners as acceptors, yielding aggregation-induced emission luminogens with tunable emission wavelength (up to 933 nm) and light absorption capability (ε up to 6.9 × 104 m–1 cm–1). notably, by integrating the spin–orbit coupling-promoted carbonyl group and the strong stretching vibrations of −cn to the d–a systems, a highly performing phototheranostic agent, namely, metic, is constructed. when encapsulating metic into nanovehicles, the obtained metic nanoparticles show excellent performance in multimodality theranostics for cancer treatment. this work is expected to provide an organic phototheranostic agent designing principle for potential clinical trials.


微信扫一扫,加设计师好友
17621261539
周一至周五8:30-18:00

提升“研值”

工作人员将在1个小时内联系您。

